Course Description
5.112 is an introductory chemistry course for students with an unusually strong background in chemistry. Knowledge of calculus equivalent to MIT course 18.01 is recommended. Emphasis is on basic principles of atomic and molecular electronic structure, thermodynamics, acid-base and redox equilibria, chemical kinetics, …
5.112 is an introductory chemistry course for students with an unusually strong background in chemistry. Knowledge of calculus equivalent to MIT course 18.01 is recommended. Emphasis is on basic principles of atomic and molecular electronic structure, thermodynamics, acid-base and redox equilibria, chemical kinetics, and catalysis. The course also covers applications of basic principles to problems in metal coordination chemistry, organic chemistry, and biological chemistry.
Course Info
Instructors
Departments
Learning Resource Types
theaters
Lecture Videos
notes
Lecture Notes
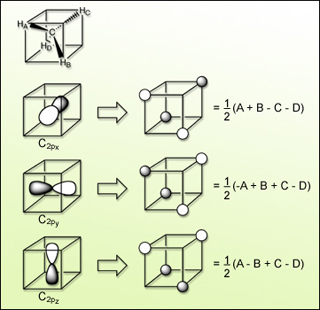
Linear combinations of H 1s atomic orbitals that match nodal properties of C 2p atomic orbitals for tetrahedral methane. (Figure by MIT OpenCourseWare.)








