Course Description
This course deals with the application of structure and theory to the study of organic reaction mechanisms: Stereochemical features including conformation and stereoelectronic effects; reaction dynamics, isotope effects and molecular orbital theory applied to pericyclic and photochemical reactions; and special reactive …
This course deals with the application of structure and theory to the study of organic reaction mechanisms: Stereochemical features including conformation and stereoelectronic effects; reaction dynamics, isotope effects and molecular orbital theory applied to pericyclic and photochemical reactions; and special reactive intermediates including carbenes, carbanions, and free radicals.
Course Info
Instructor
Departments
Topics
Learning Resource Types
grading
Exams
notes
Lecture Notes
assignment
Problem Sets
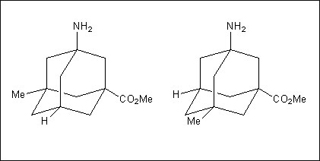
These two adamantane structures are enantiomers, or mirror images, of each other. (Image by MIT OpenCourseWare.)










