Course Description
This course focuses on the latest scientific developments and discoveries in the field of nanomechanics, the study of forces and motion on extremely tiny (10\-9 m) areas of synthetic and biological materials and structures. At this level, mechanical properties are intimately related to chemistry, physics, and quantum …
This course focuses on the latest scientific developments and discoveries in the field of nanomechanics, the study of forces and motion on extremely tiny (10\-9 m) areas of synthetic and biological materials and structures. At this level, mechanical properties are intimately related to chemistry, physics, and quantum mechanics. Most lectures will consist of a theoretical component that will then be compared to recent experimental data (case studies) in the literature. The course begins with a series of introductory lectures that describes the normal and lateral forces acting at the atomic scale. The following discussions include experimental techniques in high resolution force spectroscopy, atomistic aspects of adhesion, nanoindentation, molecular details of fracture, chemical force microscopy, elasticity of single macromolecular chains, intermolecular interactions in polymers, dynamic force spectroscopy, biomolecular bond strength measurements, and molecular motors.
Learning Resource Types
theaters
Demonstration Videos
grading
Exam Solutions
grading
Exams
notes
Lecture Notes
theaters
Simulation Videos
assignment_turned_in
Written Assignments with Examples
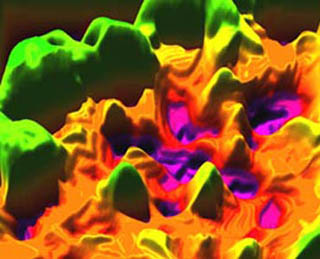
This 3D illustration of a modulus map of bone was produced using atomic force microscope (AFM) data on the nanomechanical spatial heterogeneity of bone stiffness. Simulations using this data predict markedly different biomechanical properties compared with a uniform material, which may serve as a design consideration for biologically inspired materials technologies. See Tai, K., M. Dao, S. Suresh, A. Palazoglu, and C. Ortiz. “Nanoscale Heterogeneity Promotes Energy Dissipation in Bone.” Nature Materials 6 (June 2007): 454-462. (Image by Prof. Christine Ortiz.)










