Course Description
This course applies the concepts of reaction rate, stoichiometry and equilibrium to the analysis of chemical and biological reacting systems, derivation of rate expressions from reaction mechanisms and equilibrium or steady state assumptions, design of chemical and biochemical reactors via synthesis of chemical …
This course applies the concepts of reaction rate, stoichiometry and equilibrium to the analysis of chemical and biological reacting systems, derivation of rate expressions from reaction mechanisms and equilibrium or steady state assumptions, design of chemical and biochemical reactors via synthesis of chemical kinetics, transport phenomena, and mass and energy balances. Topics covered include: chemical/biochemical pathways; enzymatic, pathway, and cell growth kinetics; batch, plug flow and well-stirred reactors for chemical reactions and cultivations of microorganisms and mammalian cells; heterogeneous and enzymatic catalysis; heat and mass transport in reactors, including diffusion to and within catalyst particles and cells or immobilized enzymes.
Course Info
Instructors
Departments
Learning Resource Types
assignment_turned_in
Problem Sets with Solutions
grading
Exams with Solutions
notes
Lecture Notes
assignment_turned_in
Activity Assignments with Examples
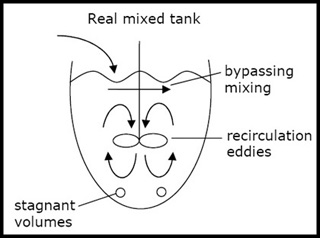
In a real mixed tank, there is not perfect mixing due to dead volumes, bypasses, and recirculation. In a real plug flow reactor or packed bed reactor, there is back-mixing, axial dispersion, and channeling. See Lec #10 for how to model these behaviors. (Figure by MIT OCW.)








