Topics
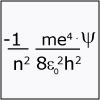
|
|
Lecture Video
The idea that matter (and thus an electron) has both particle-like and wave-like properties is introduced, and chemist Darcy Wanger Grinolds introduces us to quantum dot technology. We also start to consider the impact that the Schrödinger equation had on our understanding of the atom.
Lecture Notes
Clicker Questions
Lecture 4 Clicker Questions (PDF)
Textbook Reading
| TOPICS | 5th EDITION | 4th EDITION |
|---|---|---|
| The Wave-Particle Duality of Matter | Section 1.5 | Section 1.5 |
| The Uncertainty Principle | Section 1.6 | Section 1.6 |
Related Behind the Scenes at MIT Videos
Labeling Tumors With Quantum Dots
Quantum dots are tiny semiconductor crystals with vivid colors that can be used as visual labels in biology and medicine. Quantum dots excited by UV radiation emit light with an energy and color that is determined by the size of the quantum dot. Darcy Wanger describes how the characteristics of atomic energy levels relate to the color of quantum dots, and how quantum dots may someday be used as markers in surgical procedures.
Darcy Wanger describes her realization that science is not something “done—in the past tense” by people long ago, but rather an exciting pursuit that requires social interactions to solve current real-world questions.








