Course Description
This design course teaches a systematic approach for development of an implantable or injectable medical device to treat a specific and well-defined clinical problem, criteria for preparing applications to the US Food and Drug Administration for approval to conduct clinical trials, and the steps to start a company to …
This design course teaches a systematic approach for development of an implantable or injectable medical device to treat a specific and well-defined clinical problem, criteria for preparing applications to the US Food and Drug Administration for approval to conduct clinical trials, and the steps to start a company to make it available to the patient. Students work in teams to develop the design for an FDA Class III medical device or combination product (incorporating drugs and/or biologics). The emphasis is on the science and engineering underlying the design of novel treatments for problems in any one of the 78 organs of the body.
Course Info
Learning Resource Types
notes
Lecture Notes
theaters
Lecture Videos
menu_book
Open Textbooks
group_work
Projects
auto_stories
Readings
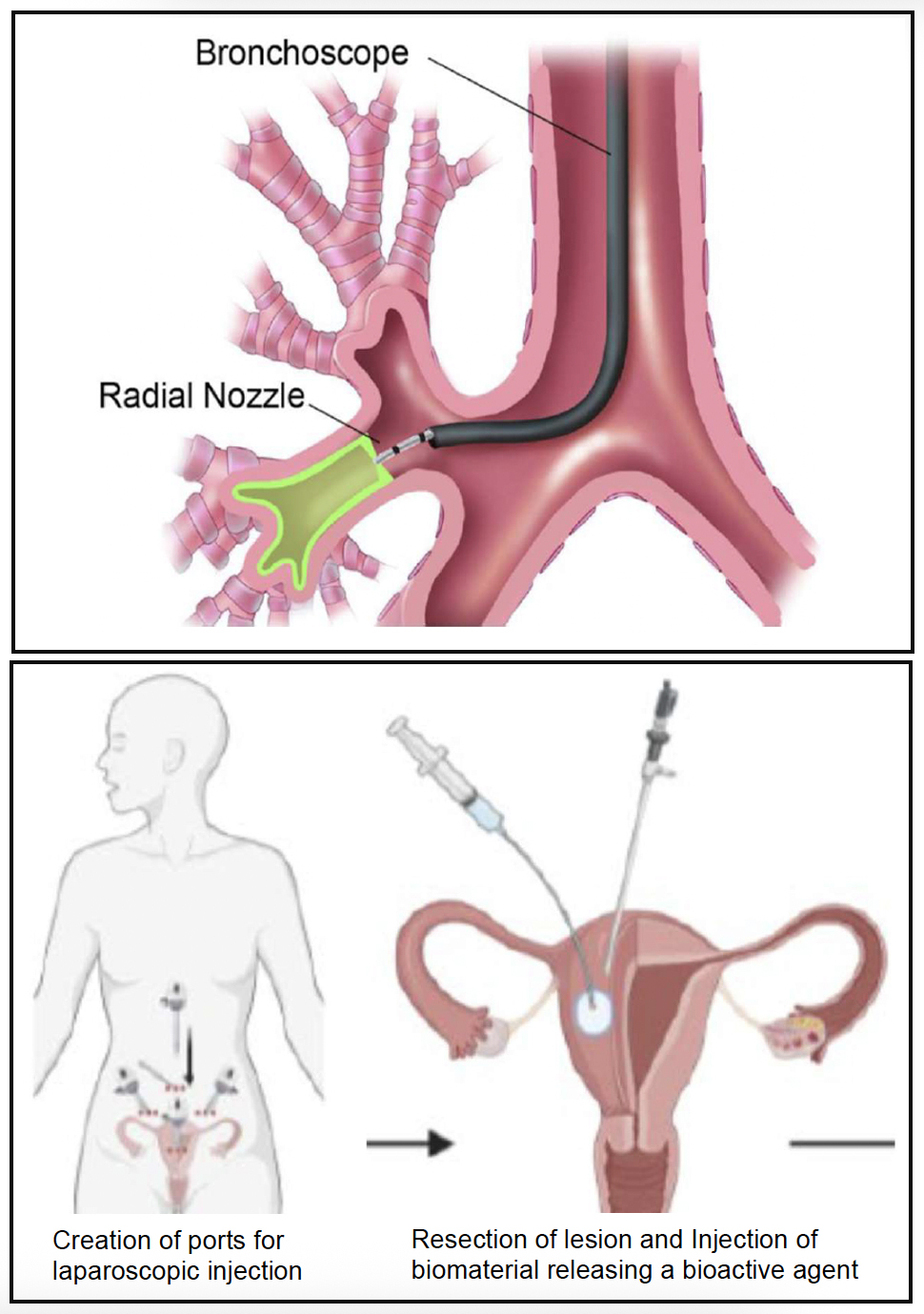
Prior 2.782J/HST.524J design projects. (Top) Endobronchial drug-eluting biomaterial for the treatment of asthmatic patients; A. Orji, B. Schelhaas, J. Xu, C.A. Luna (2020). (Bottom) Injectable biomaterial for prevention of post-surgical recurrence of superficial peritoneal endometriosis and fibrosis; P. Chacko, J. Komen, J. Lee, A. Seabold (2023). (Image by Prof. Myron Spector.)










