Course Description
The theoretical frameworks of Hartree-Fock theory and density functional theory are presented in this course as approximate methods to solve the many-electron problem. A variety of ways to incorporate electron correlation are discussed. The application of these techniques to calculate the reactivity and spectroscopic …
The theoretical frameworks of Hartree-Fock theory and density functional theory are presented in this course as approximate methods to solve the many-electron problem. A variety of ways to incorporate electron correlation are discussed. The application of these techniques to calculate the reactivity and spectroscopic properties of chemical systems, in addition to the thermodynamics and kinetics of chemical processes, is emphasized. This course also focuses on cutting edge methods to sample complex hypersurfaces, for reactions in liquids, catalysts and biological systems.
Learning Resource Types
notes
Lecture Notes
assignment
Written Assignments
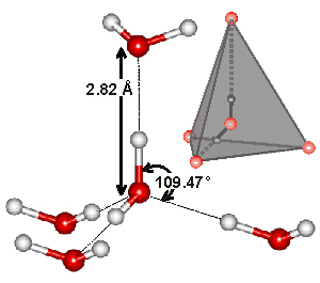
Tetrahedral H-bonded water pentamer figure, O-O 0.282 nm, O–O 0.282 nm, O-O-O 109.47°. From Hydrogen Bonding in Water. (Image courtesy of Professor Martin Chaplin, London South Bank University. Used with permission.)








