Course Description
This course provides an introduction to the physical chemistry of biological systems. Topics include: connection of macroscopic thermodynamic properties to microscopic molecular properties using statistical mechanics, chemical potentials, equilibrium states, binding cooperativity, behavior of macromolecules in solution …
This course provides an introduction to the physical chemistry of biological systems. Topics include: connection of macroscopic thermodynamic properties to microscopic molecular properties using statistical mechanics, chemical potentials, equilibrium states, binding cooperativity, behavior of macromolecules in solution and at interfaces, and solvation. Example problems include protein structure, genomic analysis, single molecule biomechanics, and biomaterials.
Course Info
Learning Resource Types
grading
Exams
assignment
Problem Sets
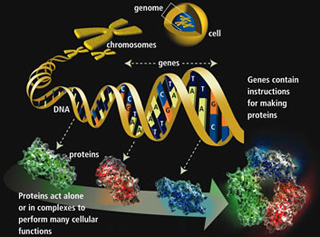
Genes are specific sequences of bases that encode instructions on how to make proteins. It’s the proteins that perform most life functions and even make up the majority of cellular structures. Proteins are large, complex molecules made up of smaller subunits called amino acids. (Illustration courtesy of the Human Genome Program of the U.S. Department of Energy.)










