Course Description
This course is a self-contained concise review of general thermodynamics concepts, multicomponent equilibrium properties, chemical equilibrium, electrochemical potentials, and chemical kinetics, as needed to introduce the methods of nonequilibrium thermodynamics and to provide a unified understanding of phase …
This course is a self-contained concise review of general thermodynamics concepts, multicomponent equilibrium properties, chemical equilibrium, electrochemical potentials, and chemical kinetics, as needed to introduce the methods of nonequilibrium thermodynamics and to provide a unified understanding of phase equilibria, transport, and nonequilibrium phenomena useful for future energy and climate engineering technologies. Applications include second-law efficiencies and methods to allocate primary energy consumptions and CO₂ emissions in cogeneration and hybrid power systems, minimum work of separation, maximum work of mixing, osmotic pressure and membrane equilibria, metastable states, spinodal decomposition, and Onsager’s near-equilibrium reciprocity in thermodiffusive, thermoelectric, and electrokinetic cross effects.
Course Info
Learning Resource Types
grading
Exams
Instructor Insights
notes
Lecture Notes
theaters
Lecture Videos
assignment_turned_in
Multiple Assignment Types with Solutions
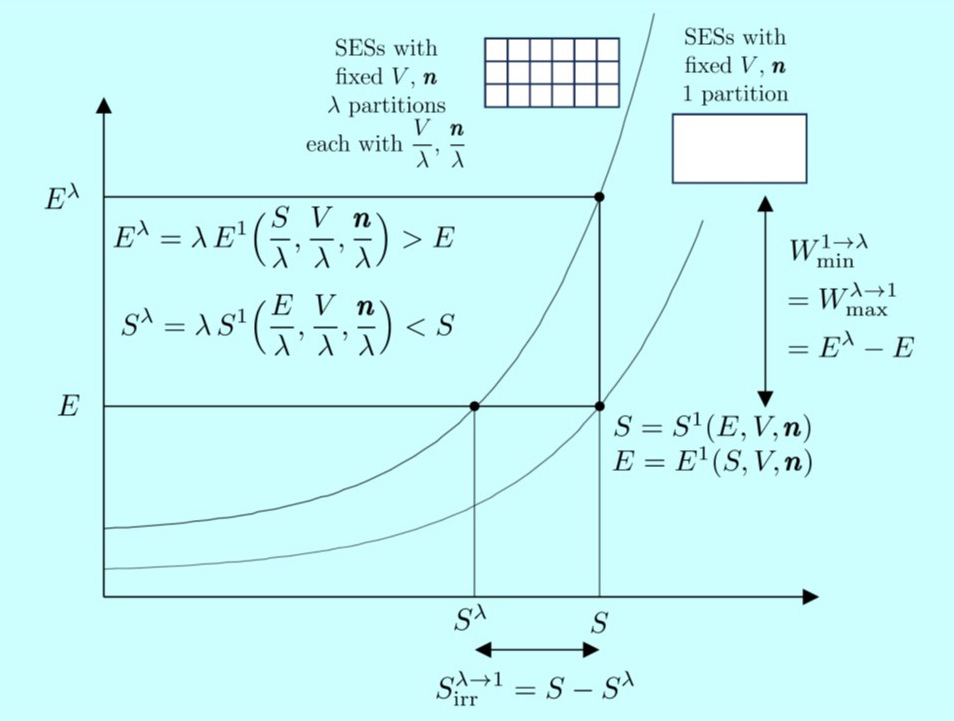
Energy versus entropy diagrams are used extensively in this course to illustrate effectively many fundamental concepts about nonequilibrium states as well as thermodynamic equilibrium. This picture is used in lecture 9 to show the important role played by wall rarefaction effects in determining thermodynamic equilibrium properties of small systems. (Image courtesy of Prof. Gian Paolo Beretta.)










