2.2 - Expert Experimentalist Rating
“Acid, Base, and in Between”
Techniques Checklist
- Separation of multi-component mixture using pKa
- Planning an extraction and washing sequence
- Careful transfer of solutions without loss of material
- Solvent drying and concentration
- Melting point determination
Pre-Lab Discussion and Required Reading
- Same as CC
Digital Lab Techniques Manual
- Video 5, Reaction Work-Up I: Extracting, Washing & Drying
- Video 6, Reaction Work-Up II: Using the Rotavap
- Video 11, Using a Balance
- Video 12, Melting Point Determination
Equipment
- Graduated Cylinder (100-mL)
- Separatory funnel (125-mL)
- Erlenmeyer flasks (4x250-mL)
- Beaker (150-mL)
- Round-bottomed flask (100-mL)
- pH paper
- NMR tube
- Funnel
- Filter paper
- Rotary evaporator
Goal
To separate a three-component mixture using differences in pKa, with minimal loss of material.
Experiment Outline
You will receive a vial containing 100 mg each of benzoic acid, 4-nitroaniline, and naphthalene. Using the pKa, values of these molecules, carefully devise an extraction and washing sequence that will selectively separate the three components.1
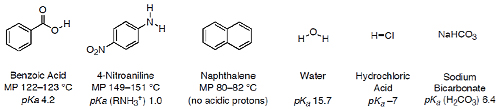
- Before beginning your extraction sequence, check with your TA or professor to make sure that it will work. You are free to use any or all of the following solvents:
- Diethyl Ether
- Methanol
- Water
- Saturated Sodium Bicarbonate Solution (Aqueous)
- 6 M HCl
- 1 M NaOH
- Carry out your extraction and washing sequence, isolating each of the three components.
- For each compound, remove the solvent by rotary evaporation to a constant weight and obtain a mass.
- Obtain a melting point for each compound.
Results
To obtain your “EE Rating” in Transfer and Extraction Techniques, you must isolate at least 90 mg of two of the three compounds. In addition, the isolated compounds should melt over no more than three degrees, with the range beginning no lower than two degrees below the melting point values given above. 1Adapted from Gilbert, J. C., and S. F. Martin. Experimental Organic Chemistry: A Miniscale & Macroscale Approach. 3rd ed. Pacific Grove, p. 141.










