5.1 - Competent Chemist Rating
“Looks Are Sometimes Deceiving”
Techniques Checklist
- Analyzing mixtures by TLC
- Assembling a silica gel column
- Applying crude mixtures to a silica gel column
- Separating simple mixtures with a silica gel column
Pre-lab Discussion
- Theory of column chromatography—Reading Zubrick chapter 28, Mohrig chapter 18
- TLC—polarity/solvent sys—Reading Zubrick chapter 27, LLP chapter 9.3.1, Mohrig chapter 17
- Setting up silica gel column Reading Zubrick chapter 31, LLP chapter 11.6, Mohrig chapter 18
- Applying crude mixtures to the column
- Running a flash column
Digital Lab Techniques Manual
- Video 3, TLC: The Basics
- Video 10, Column Chromatography
Equipment
- Flash Chromatography Column
- Air Flow apparatus (Stopper, T-valve, Screw clamp, tubing)
- Round-bottomed flasks—1x100-mL, 2x500-mL
- Test tubes—18x150 mm
- Test tube racks
- TLC plates—cut silica/glass plates and UV lamp
- Large plastic funnels
Goal
- Purify a contaminated compound using silica gel flash column chromatography.
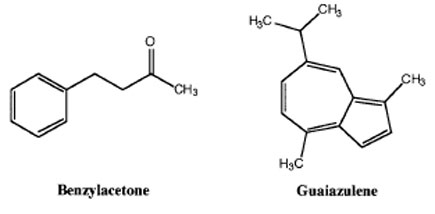
Experiment Outline
- You will be given 2 mL of an ether/pentane solution containing 1.00 g of benzylacetone contaminated with a small amount of guaiazulene.
- Analyze this mixture by TLC—see TLC Guide, using 10% ethyl acetate/hexanes as the solvent system.
- Record the Rf values.
- Prepare the column in the hood, using 10% ether/pentane and 50 g (about 5’’) of of silica gel—see Flash Column Chromatography Guide.
- Elute the column with 10mL of pentane—Apply your sample to the column, being careful not to disturb the top layer of sand. Rinse the sample flask three times with 1 mL pentane each, and use the rinses to wash the sides of the column.
- Run the column, monitoring the fractions by TLC—See Flash Chromatography Guide and TLC Guide.
- Concentrate the set of fractions containing pure benzylacetone.
- Weigh the purified compound and prepare a GC and GC-MS sample.
- Check product with TLC and obtain a GC and GC-MS spectrum.
Results
- To obtain your “CC Rating” in Purification by Flash Column Chromatography, you should collect at least 0.95 g of benzylacetone. This sample must be at least 95% pure as demonstrated by GC spectroscopy. Your sample must also be submitted to the TA for possible weight and purity verification.
5.2 - Expert Experimentalist
Techniques Checklist
- Picking the correct eluent then adsorbtion of a crude mixture onto silica gel
- Separating complex mixtures—using gradient elution
Pre-lab Discussion
- Suggest limited list of eluent solvent systems
- Discuss sample adsorption and gradient elution strategies
Equipment
- Identical to CC Level
Goal
Separate mixture of three compounds using gradient elution flash column chromatography.
Chemical Data
- Benzylacetone—FW 148.21, bp 235 °C, d 0.989
- Benzylideneacetone—FW 146.19, mp 39–41 °C
- 3-Methylanisole—FW 122.17, bp 175–176 °C, d 0.969
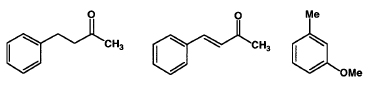
Experiment Outline
- You will be given 1.00 g of a mixture containing 0.60 g of the major ketone, 0.20g of the minor ketone, and 0.20g of methylanisole in 20 ml of an ether/ hexane solution.
- Analyze this mixture by TLC using various solvent systems—see TLC Guide for hints.
- Pick an eluent.
- Decide on the silica gel to compound ratio.
- Prepare the column.
- Deposit the mixture on silica gel, dry completely, then apply to the column.
- Run the column.
- Concentrate pure fractions.
- Weigh the purified compounds.
- Analyze the pure ketones by NMR and TLC.
Note
- The ketones are somewhat volatile, and 3-methylanisole, with its low molecular weight, is much more so. Therefore, do not concentrate it (or a mixture containing it) using the vacuum pump.
Results
- To obtain your “EE Rating” in Purification by Flash Column Chromatography, you should get at least 0.45 g of the major ketone and 0.13 g of the minor ketone, and 0.13 g of methylanisole. These samples must be at least 95% pure as demonstrated by NMR spectroscopy. Your samples must also be submitted to the TA for possible weight and purity verification.








