4.1 - Competent Chemist Rating
“How Did the Peach Get Inside the Banana?”
Techniques Checklist
- Setting up distillation glassware correctly
- Performing atmospheric pressure distillations
- Using Gas Chromatography and Mass Spectrometry GC-MS to analyze samples
Pre-lab Discussion
- Theory of distillation—Reading: Zubrick chapter 34, LLP chapter 11.3, Mohrig chapter 13
- Distillation glassware and how to set it up—Reading: Zubrick chapter 19
- Use of the GC—Reading: Zubrick chapter 30, GC-MS- Mohrig chapter 19
Digital Lab Techniques Manual
- Video 11, Using a balance
- Video 15, Distillation I
Equipment
- 25-mL & 50-mL round-bottomed flask with stir bar
- 3 Scintillation Vials or flasks that fit short path
- Distillation Kits (distillation head)
- Ground glass Thermometer and adapter
- Keck clips
- Glass wool and aluminum foil (optional)
- Heating mantle w/sand
- Variac
Goal
- To purify a mixture of two liquids using distillation.
Experiment Outline
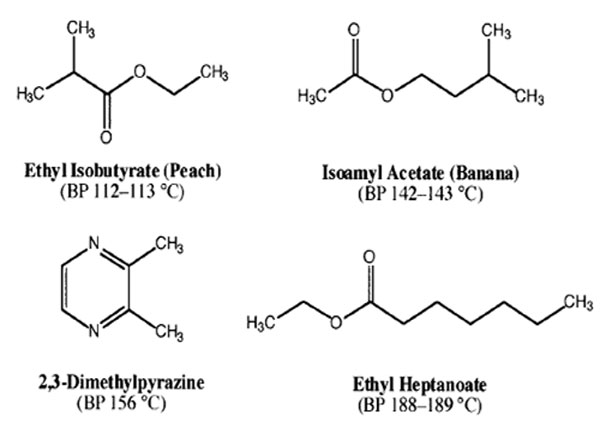
- You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °C (See possible compounds below).
- Analyze the mixture using the GC—see GC Sample Preparation and GC Operation Guides.
- Perform atmospheric pressure distillation—see Distillation Guide.
- Prepare a GC sample of your purified, low-boiling fraction.
- Obtain a mass spectrum and a gas chromatogram of your purified low-boiling compound.
Helpful Hints
- Make sure all your joints are lightly greased and sealed well. Otherwise, you will lose your product into the atmosphere.
- Do not heat your mixture too fast, or your entire sample may end up in your collection flask.
- Insulate your distillation head with cotton and foil to increase the rate of distillation.
- Be aware that the temperature reading on the thermometer may not correlate accurately with the boiling point of the distilling liquid.
Results
- To obtain your “CC Rating” in Purification of Liquids by Distillation, you must obtain at least 7.00 g of the low-boiling material that is 92% pure or better, as determined using GC analysis. You must also correctly identify the two components of your mixture. Think boiling points and smell.
4.2 - Expert Experimentalist Rating
“What’s With Those High-Altitude Recipes Anyway?”
Techniques Checklist
- Glassware setup for reduced pressure distillation
- Running reduced pressure distillation
Pre-lab Discussion
- Differences between atmospheric pressure and reduced pressure distillation
Digital Lab Techniques Manual
- Video 16, Distillation II
Equipment
- 25-mL Round-bottomed flask
- 3x10-mL Pear-shaped flasks
- Vigreux column-Vacuum Distillation Kits
- Short path distillation head
- Ground glass Thermometer and cow adapter
- Keck clips
- Glass wool and aluminum foil
- Heating Mantle (w/sand) and Variac
Goal
- To purify a mixture of two liquids by reduced pressure distillation.
Experiment Outline
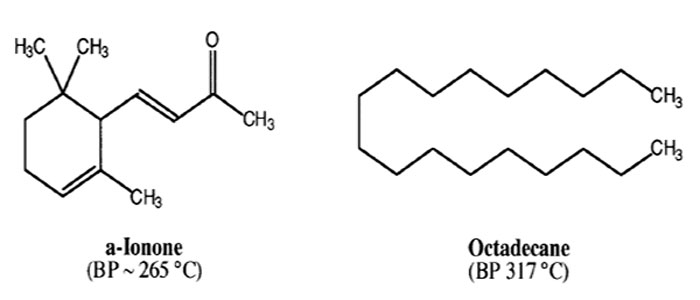
- You will receive a vial containing 7.50g of a mixture of alpha-ionone and octadecane. Repeat procedure for CC level distillation but using a Vigreux column and the vacuum line—see Distillation Guide.
Results
- To obtain your “EE Rating” in Purification of Liquids by Distillation you must predict the boiling points of the compounds in your mixture at 0.5 torr. You must also obtain at least 4.00 g of alpha-ionone that is 93% pure or better as determined using GC analysis.
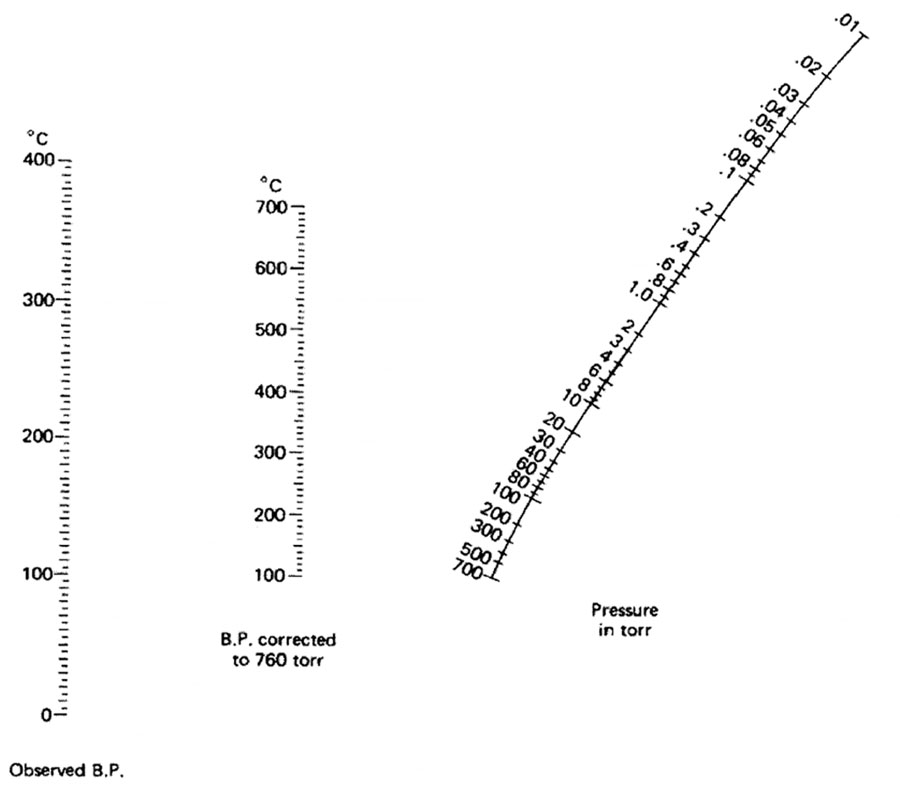
The picture below is a nomograph. using it and a ruler, you can determine at what temperature a liquid will boil under vacuum.