3.1 - Competent Chemist Rating
“How Do You Recrystallize a Mothball?”
Techniques Checklist
- Solubility tests
- Choosing a good solvent system
- Decolorization
- Inducing crystallization
- Filtration
Pre-Lab Discussion
- Theory of recrystallization Reading: Zubrick chapter 13; LLP chapter 11.2, Mohrig chapter 15
Equipment
- Test tubes—five 13x100 mm
- 2x50-mL 1x 125-mL Erlenmeyer flasks
- Small magnetic stir bars
- Stemless funnel and fluted filter paper
- Büchner funnel and filter paper
- Magnetic stirring/hot plate
- 250-mL Filter flask and aspirator stopper
- Rubber filter adapters
- Large vial with white cap
- Solid 7 rubber stopper
- Test tube rack
- Large crystallizing dish / Desiccator
Goal
You will be given 2.00 g of impure naphthalene (mothballs!); your job is to purify the naphthalene by recrystallization without losing a significant amount of your sample!1
Experiment Outline
Part I: Solubility Tests
Determine an appropriate solvent system for the recrystallization of naphthalene. For your tests try: water, methanol, acetone, hexane, and toluene. To understand how to find the appropriate solvent or solvent mixture for recrystallization, see Zubrick or Mohrig.
Part II: Recrystallization of Naphthalene
- Transfer the material to a 50-mL Erlenmeyer flask equipped with a stir bar. Add about 20 mL of the solvent (determined in Part I) and heat to boiling on a stir/hot plate.
- Remove any insoluble impurities by filtration, and recrystallize your product—see Two-Solvent Recrystallization Guide.
- Collect your crystals on a small Büchner funnel by vacuum filtration, and rinse with the cold solvent mixture.
- Your crystals should be colorless. If some orange or yellow color persists, wash your material with cold hexane. (Be careful: What is the solubility of naphthalene in hexane?).
- Dry your compound well—see Two-Solvent Recrystallization Guide for tips.
- Determine the yield and obtain a melting point.
Results
To obtain your “CC Rating” in Purification of Solids by Crystallization, you must obtain colorless crystals (no traces of yellow) weighing at least 1.30 g (well dried!) and melting over no more than three degrees with the lower range beginning no lower than 77 °C and the upper range ending no higher than 83 °C. This material must also be submitted to the TA for possible weight and melting point verification.
3.2 - Expert Experimentalist Rating
“The Single-Crystal Shakedown”
Overview
X-Ray diffraction is an important and powerful tool for determining the solid state structure of compounds. Modern advances have made data collection and structure solution almost routine for many small molecules. To use this technique, however, good quality single crystals are still needed. In this exercise, you will experiment with the art of growing single crystals.
Techniques Checklist
- Manipulation of milligram quantities of material
- Syringe use
- Crystallization techniques for growing good quality single crystals
Pre-Lab Discussion
- Use of different recrystallization techniques: vapor diffusion, solvent layering, temperature variation
Digital Lab Techniques Manual
- Video 17, Refluxing a Reaction
Equipment
- Magnetic stirring hot plate
- 50-mL Round-bottomed flask
- Condenser
- Stir bar
- Vials (3 large, 4 small) + 2 Glass jars
- Glass frit (D)
- Side-arm 250 mL Erlenmeyer flask / rubber filter adaptor
- 2-mL Glass syringe
- Variac and heating mantle
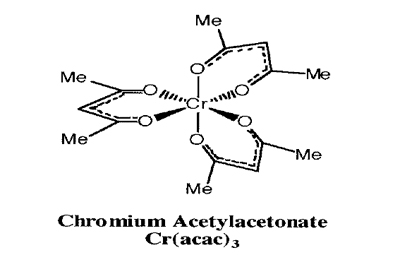
Goal
Synthesize Cr(acac)3,2 then perform several slow recrystallizations to obtain a single crystal of satisfactory quality.
Experiment Outline
Before coming to the lab, perform the necessary calculations to fill in the following table.
| REAGENT | SOURCE | FORM. WT. | DENSITY | MASS/VOL. | MMOLES | EQUIV |
|---|---|---|---|---|---|---|
| CrCl3·6H2O | 1.00 mmol | 1 | ||||
| Urea | 17 | |||||
| 2,4-pentanedione | 8 | |||||
| Cr(acac)3 | Product |
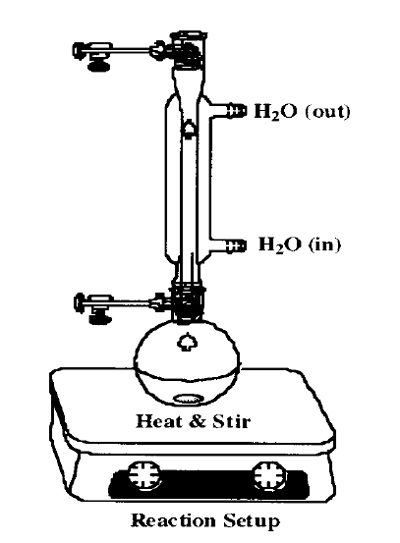
Experimental
- Dissolve CrCl3·6H2O in 2 mL of distilled water in a 50-mL round-bottomed flask, equipped with a stir bar.
- Add the urea in one portion to the flask, and stir until completely dissolved.
- Add the 2,4-pentanedione dropwise via syringe.
- Attach the condenser to the flask, and heat the mixture to vigorous reflux (this is important!), with stirring, for about 1 hour.
- Cool the reaction flask to room temperature, and collect the product by vacuum filtration on a size D glass frit funnel, washing with cold water.
- Dry the product in vacuo (using the high-vac. desiccator provided by the TAs), and obtain a yield or dry the product in your desiccator overnight, and obtain a yield and melting point of the product.
- Set up multiple crystallizations to grow single crystals—see Growing a Single Crystal Guide.
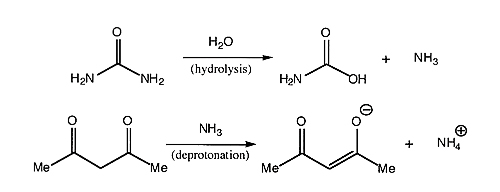
Note
- Urea slowly hydrolyzes in the acidic solution used for this reaction, liberating ammonia (NH3), which controls the pH of the reaction. As more NH3 is generated, the solution becomes more basic, making it easier to remove the proton from the acac (acetylacetonate, also known as 2,4-pentanedione); it is the acac anion which then coordinates to the metal to form the desired Cr(acac)3 complex. What is the limiting reagent? Calculate your percent yield.
Helpful Hints
When using a saturated solution to grow crystals, filter the solution through a plug of glass wool in a pipette before setting up the crystallization.
Results
- To obtain your “EE Rating,” you must obtain ≥ 45% yield of Cr(acac)3 and you must produce at least one single crystal that is suitable for X-ray analysis.
1Adapted from Fieser, L. F., and K. L. Williamson. Organic Experiments. 7th ed. D. C. Heath and Company, 1992, p. 40.
2Adapted from Szafran, Z., R. M. Pike, and M. M. Sing. “Synthesis of Metal Acetylacetonates.” In Microscale Inorganic Chemistry: A Comprehensive Laboratory Experience. Wiley, 1991, pp. 224–9.








