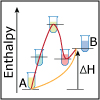
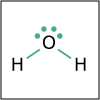
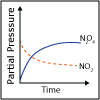
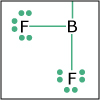
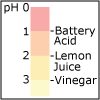
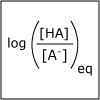
Using the description of the atom and of atomic properties from Unit I and using knowledge about bonding and molecular structure from Unit II, in Unit III we investigate how, why, and when molecules react with each other. Will they or Won’t they? That is the question that thermodynamics answers for us. Viewers of this unit will be introduced to Gibbs free energy, enthalpy and entropy, and will learn the key to spontaneity. Most reactions are reversible and thus understanding chemical equilibrium is of the utmost importance. What will the ratio of products to reactants be at equilibrium? How can this ratio be manipulated by factors such as temperature or pressure? By the end of this unit, viewers will have the tools needed to work even the most challenging of chemical equilibrium problems, i.e. those that involve acids and bases. Viewers will be able to design a strategy for shifting the equilibrium of a reaction to make a desired product. They will have a working knowledge of thermodynamics and equilibrium such that they can apply that knowledge to other disciplines, and be ready to help solve real world problems like climate change and the development of new medical therapies.
|
|
|
|
|
|
Looking for something specific in this course? The Resource Index compiles links to most course resources in a single page.