Topics
|
|
Lecture Video
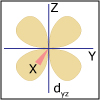
Crystal field theory was developed to explain the special features of transition metal complexes, including their beautiful colors and their magnetic properties. In part I of this topic, we consider d-block coordination complexes that have octahedral geometry, and see whether we can change the color of a paper flower dipped in an octahedral cobalt chloride complex just by adding water.
Lecture Notes
Clicker Questions
Lecture 28 Clicker Questions (PDF)
Textbook Reading
| TOPICS | 5th EDITION | 4th EDITION |
|---|---|---|
| The Electronic Structures of Complexes | Sections 16.8–16.11 | Sections 16.8–16.11 |








