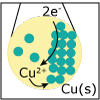
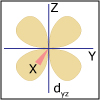
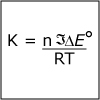In Unit IV, we continue to consider chemical reactions but now focus on reactions that involve the loss (oxidation) and gain (reduction) of electrons. Whereas metals in group I and II of the periodic table are only oxidized by one and two electrons, respectively, transition (d-block) metals are capable of achieving multiple oxidation states. Revisiting the concept of atomic orbitals, we discuss how the occupation of electrons in d-orbitals gives rise to the special properties of d-block metal-containing complexes. Viewers can observe some of these special properties for themselves as spectacular color changes afforded by solutions of nickel and cobalt salts are demonstrated. By the end of the unit, viewers should be able to calculate electrochemical cell potentials, identity which compound is the better oxidizing agent, draw energy diagrams that explain why one cobalt compound is blue and another red, and explain how a chelator works to treat acute lead poisoning.
Looking for something specific in this course? The Resource Index compiles links to most course resources in a single page.