Topics
|
|
Lecture Video
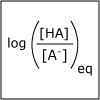
A buffer helps to maintain a constant pH. Our blood has a natural buffering system to ensure that the pH of our blood stays within a narrow window and that we stay health. In this lecture, we consider how to design a buffer. We also discuss how one can predict the pH of a salt solution. At dinner if you put table salt in your water glass, how would the pH of the water change? or would it change?
Lecture Notes
Clicker Questions
Lecture 22 Clicker Questions (PDF - 1.1MB)
Textbook Reading
| TOPICS | 5th EDITION | 4th EDITION |
|---|---|---|
| Autoprotolysis and pH | Sections 11.13 and 11.18–11.19 | Sections 10.13 and 10.18–10.19 |
| Mixed Solutions and Buffers | Sections 12.1–12.3 | Sections 11.1–11.3 |










