Topics
|
|
Lecture Video
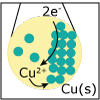
Redox reactions are a major class of chemical reactions in which there is an exchange of electrons from one species to another. In this lecture, the basic principles of redox reactions are introduced. If you want to design the next greatest battery to power your favorite electronic device, you won’t want to miss this lecture.
Lecture Notes
Clicker Questions
Lecture 25 Clicker Questions (PDF)
Textbook Reading
| TOPICS | 5th EDITION | 4th EDITION |
|---|---|---|
| Galvanic and Electrolytic Cells | Sections 13.3–13.12 | Sections 12.3–12.12 |










